Clinical Trials: What They Are and Why They Matter
When working with Clinical Trials, systematic studies that evaluate medical interventions in human participants to determine safety, efficacy, and optimal use. Also known as human research studies, it provides the evidence base for modern healthcare. Clinical trials are the bridge between laboratory discoveries and everyday treatments, and they follow a structured pathway that starts with a clear research question and ends with regulatory decisions.
One of the most common designs you’ll see is the Randomized Controlled Trial, a study where participants are randomly assigned to receive either the experimental therapy or a comparator such as a placebo. This randomization reduces bias and helps researchers attribute outcomes directly to the intervention. The use of a Placebo, an inactive substance that mimics the appearance of the active drug, allows the trial to measure the true effect size by comparing it to a group that receives no therapeutic benefit. Both concepts are essential because they influence the reliability of results and shape the next steps in drug development.
Beyond study design, successful clinical trials depend on Patient Recruitment, the process of identifying, screening, and enrolling suitable volunteers. Without a sufficient and diverse participant pool, a trial can’t generate meaningful data, which in turn stalls Regulatory Approval, the authorization granted by agencies like the FDA or EMA after reviewing trial outcomes. Ethical oversight, typically provided by an Institutional Review Board, ensures that participants’ rights are protected throughout the study. Together, these elements encompass the full lifecycle of a clinical trial: from hypothesis, through rigorous testing, to market authorization.
What You’ll Find Below
Below you’ll discover a curated collection of articles that break down specific trial topics—such as comparing antibiotics, evaluating diabetes medications, and understanding the impact of obesity on health conditions. Each piece ties back to the core principles of trial design, safety monitoring, and real‑world application, giving you practical insights you can use right away. Dive in to see how clinical evidence is built, how it shapes treatment choices, and what the latest research says about the drugs and therapies you care about.
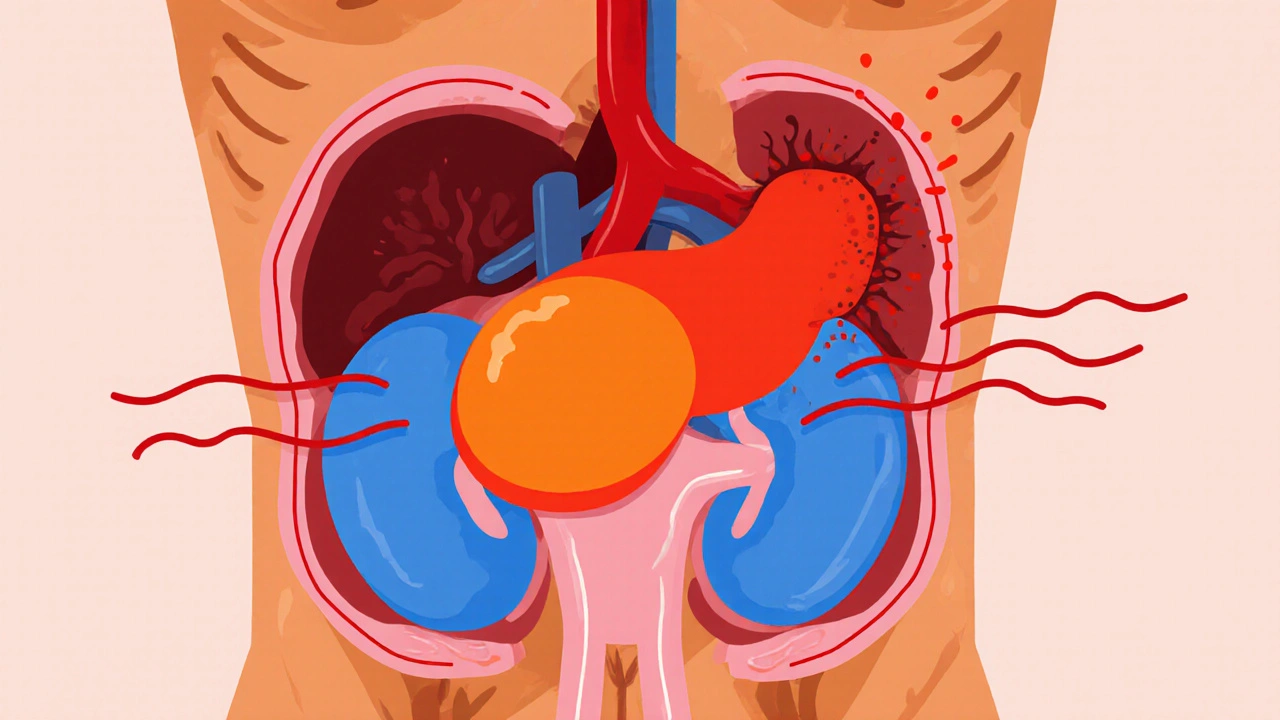
Pheochromocytoma: New Research & Treatment Advances 2025
- Oct, 18 2025
- Daniel Remedios
- 15 Comments
A concise guide on pheochromocytoma covering new genetic insights, modern diagnostics, treatment options from surgery to radiotherapy, and emerging therapies in 2025.
