When you hear the word biosimilars, you might think they’re just generic versions of expensive biologic drugs. But that’s not quite right. Biosimilars aren’t chemically identical copies like the pills you pick up at the pharmacy. They’re complex medicines made from living cells - yeast, bacteria, or animal tissue - and even tiny changes in how they’re made can affect how they work. Still, they’re designed to work just like the original biologic, and they’re saving patients and the healthcare system a lot of money.
What Exactly Are Biosimilars?
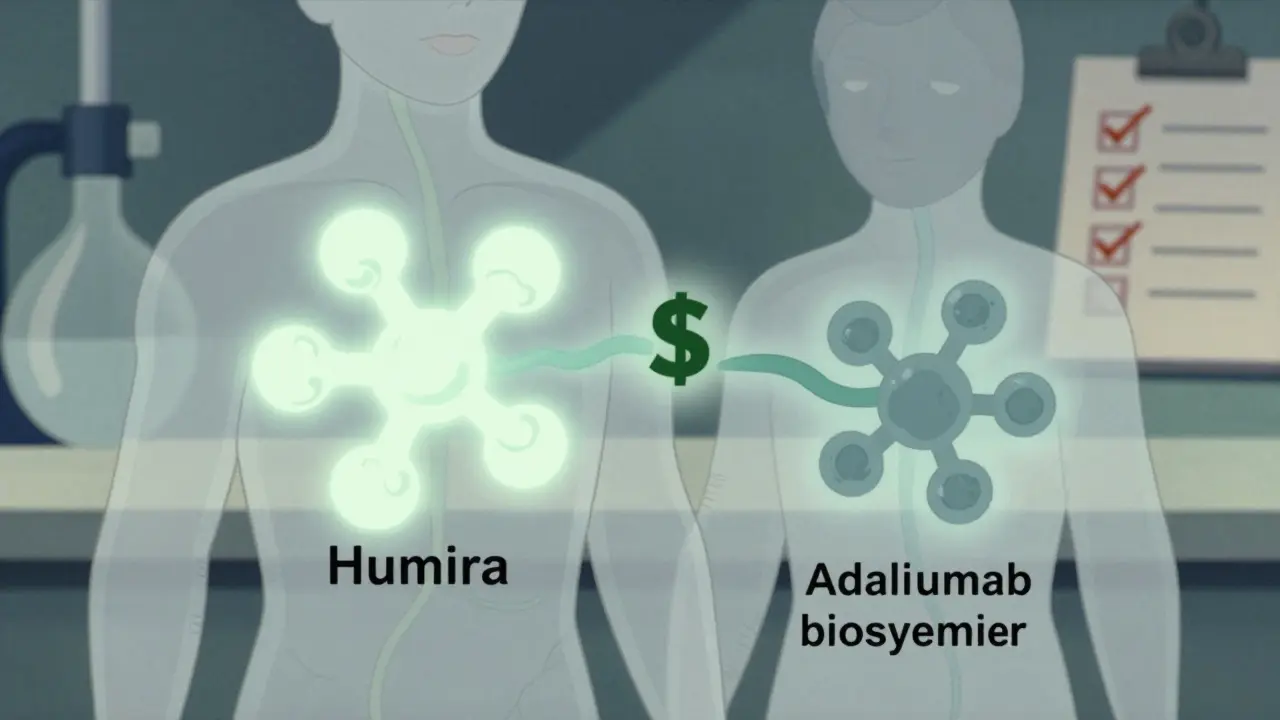
A biosimilar is a biologic medicine that is highly similar to an already approved reference product. The U.S. Food and Drug Administration (FDA) requires manufacturers to prove, through thousands of lab tests and clinical studies, that there are no clinically meaningful differences in safety, purity, or potency. That means if you’re being treated for rheumatoid arthritis, Crohn’s disease, or cancer with a biologic like Humira or Enbrel, a biosimilar will deliver the same results.
Unlike traditional generics - which are simple chemical copies of small-molecule drugs - biosimilars can’t be exact duplicates. The manufacturing process involves living organisms, and even minor changes in temperature, pH, or cell culture conditions can create small differences. But these differences aren’t harmful. The FDA’s approval process is strict: it looks at the molecule’s structure, how it behaves in the body, how the immune system reacts to it, and whether it works the same way in real patients.
The first biosimilar approved in the U.S. was Zarxio, a copy of filgrastim (used to boost white blood cells after chemotherapy), cleared by the FDA in 2015. Since then, 45 biosimilars have been approved, covering treatments for cancer, autoimmune diseases, and diabetes. The European Medicines Agency (EMA) approved its first biosimilar back in 2006, and over 16 years of real-world use have shown no unexpected safety issues.
How Do Biosimilars Save Money?
Biosimilars don’t slash prices the way generics do. While generics often cut costs by 80-85%, biosimilars typically start at 15-30% lower than the reference product. That might not sound like much, but when you’re talking about drugs that cost $7,000 a month - like Humira - even a 25% reduction means $1,750 saved per patient, per month.
In 2023, when the first Humira biosimilars hit the market, their list prices were around $5,054 - 28% lower than Humira’s $7,000. That’s not just a discount; it’s a game-changer for patients paying out-of-pocket or for insurers covering thousands of users. The Express Scripts 2023 Drug Trend Report showed that biosimilars for infliximab (Remicade) captured 72% of new prescriptions within 18 months of launch, meaning most new patients are now starting on a cheaper version.
But here’s the catch: list price doesn’t always equal what you pay. Pharmacy benefit managers, insurers, and drug manufacturers often negotiate rebates and discounts behind closed doors. That’s why some patients don’t feel the savings right away - their insurance might still be covering the brand-name drug at a high copay. The Inflation Reduction Act of 2022 changed that for Medicare Part D: starting in 2024, biosimilars will cost patients just 25% of the price, same as generics. That’s a big step toward making savings real.
Are Biosimilars Safe and Effective?
Yes. Over 10,000 patients have been studied in clinical trials comparing biosimilars to their reference products. A landmark study called NOR-SWITCH, published in The Lancet in 2016, followed over 500 patients with inflammatory diseases who switched from a reference biologic to a biosimilar. After 52 weeks, there was no increase in side effects, loss of effectiveness, or immune reactions.
Dr. Gary Lyman, a cancer specialist at Fred Hutchinson Cancer Research Center, reviewed dozens of these studies and concluded in a 2022 JAMA Oncology editorial that biosimilars have proven equivalent in efficacy and safety. The FDA states plainly: “Biosimilars are as safe and effective as their reference products.”
Even patient surveys back this up. The Arthritis Foundation surveyed 1,200 people using biosimilars in 2022. Eighty-seven percent said they noticed no difference in how well the drug worked compared to the original. Seventy-two percent reported lower out-of-pocket costs. Only 28% had initial concerns - and most of those were eased after talking with their doctor.
On Drugs.com, patients reviewing Renflexis (a biosimilar to Remicade) gave it 4.2 out of 5 stars. Common comments: “Same effectiveness as Remicade but half the cost.” The top Reddit thread on r/rheumatology had a rheumatologist with 2,500+ karma explain: “The science supports biosimilars, but we must address patient concerns through education - I spend 10-15 minutes per patient explaining the evidence.”
Why Aren’t Biosimilars Used More?
Despite the evidence, adoption isn’t uniform. In Europe, biosimilars capture over 80% of the market for drugs like filgrastim. In the U.S., it’s closer to 65% for some drugs and as low as 28% for others. Why the gap?
One reason is patent litigation. Drugmakers use legal tactics - called “product hopping” - to delay biosimilar entry. For example, when Enbrel’s patent was set to expire, the manufacturer released a new version with a different delivery device. That reset the clock on exclusivity and pushed biosimilars back by years.
Another barrier is payer policies. Some insurance plans automatically favor the brand-name drug because they get a bigger rebate from the manufacturer. Even if a biosimilar is cheaper, the insurer might not switch unless forced to. A 2022 survey of 350 oncologists by the American Society of Clinical Oncology found that 78% needed extra training to feel confident prescribing biosimilars - mostly because they were confused by coverage rules.
Also, not all biosimilars are created equal. The FDA has two categories: “biosimilar” and “interchangeable.” Only six products had the interchangeable designation as of late 2023. An interchangeable biosimilar can be substituted at the pharmacy without the prescriber’s approval - just like a generic. But most biosimilars still require a doctor’s OK to switch. Forty-eight U.S. states have laws governing substitution, and rules vary widely. Some require the pharmacist to notify the prescriber; others require written consent from the patient.
What’s Next for Biosimilars?
The pipeline is full. Seven biosimilar applications for Stelara (ustekinumab), a $10,000-a-year drug for psoriasis and Crohn’s, are under FDA review. When they launch, they could save the system billions. The Congressional Budget Office projects that by 2030, biosimilars could save U.S. healthcare $150 billion a year - if market barriers are removed.
Also on the horizon are “biobetters” - next-generation biologics designed to be better than the original. These aren’t biosimilars; they’re improved versions with longer-lasting effects or fewer side effects. They’ll compete with both the original and biosimilars, adding more complexity - and more options - to the market.
The global biosimilars market was worth $9.3 billion in 2022 and is projected to hit $33.3 billion by 2028. That growth will come from new approvals, expanded use in developing countries, and better reimbursement policies.

What Should You Do as a Patient?
If you’re on a biologic drug and your doctor suggests switching to a biosimilar, ask questions:
- Is this biosimilar approved as interchangeable?
- Has it been used in patients like me?
- Will my insurance cover it at a lower cost?
- What happens if I switch back?
Don’t assume biosimilars are riskier. The data says otherwise. But do demand transparency. If your pharmacy tries to substitute without telling you, speak up. You have the right to know what you’re getting.
Use trusted resources: the FDA’s Purple Book lists all approved biosimilars and their reference products. The Biosimilars Council offers disease-specific guides. And if you’re unsure, talk to your pharmacist or provider - they’re trained to help you navigate this.
Final Thoughts
Biosimilars aren’t a gimmick. They’re a proven, science-backed way to make life-saving treatments more affordable. They’re not perfect - market forces still hold back full adoption - but they’re working. Patients are saving money. Doctors are prescribing them. And regulators are standing behind them.
As more biologics lose patent protection over the next few years, biosimilars will become the new standard. The real challenge isn’t the science. It’s making sure the system - insurers, pharmacies, and policymakers - catches up.
Are biosimilars the same as generic drugs?
No. Generic drugs are chemically identical copies of small-molecule drugs, like aspirin or statins. Biosimilars are copies of complex biologic drugs made from living cells. Because biologics are large, intricate molecules, biosimilars can’t be exact duplicates - but they’re designed to work the same way with no clinically meaningful differences in safety or effectiveness.
Do biosimilars cause more side effects than the original biologic?
No. Multiple large studies, including the NOR-SWITCH trial and FDA-monitored post-market data, show no increase in side effects or immune reactions when switching to a biosimilar. The FDA requires extensive testing to ensure safety before approval. Real-world data from Europe and the U.S. confirms this over more than a decade of use.
Why are biosimilars cheaper if they’re so complex to make?
Because they don’t have to repeat the full clinical trials the original drug went through. Manufacturers only need to prove similarity, not start from scratch. This cuts development time and cost by 60-70%. That savings gets passed on - though not always directly to patients due to insurance and rebate structures.
Can my pharmacist switch my drug to a biosimilar without my doctor’s approval?
Only if the biosimilar is designated as “interchangeable” by the FDA AND your state allows automatic substitution. As of 2023, only six biosimilars have that status. In 48 states, laws require either the prescriber’s consent or notification before substitution. Always check with your pharmacist and doctor before any switch.
Will switching to a biosimilar affect my treatment outcome?
For most patients, no. Studies show equivalent effectiveness and safety. A 2022 Arthritis Foundation survey found 87% of users reported no difference in symptom control. However, if you’ve been stable on a biologic for years, some doctors prefer to keep you on it unless there’s a clear cost or access benefit. Always discuss switching with your provider.
How do I find out if a biosimilar is available for my medication?
Check the FDA’s Purple Book, which lists all approved biosimilars and their reference products. You can also ask your doctor or pharmacist, or visit the Biosimilars Council website. If your drug is a high-cost biologic like Humira, Enbrel, or Stelara, biosimilars are likely already available or coming soon.

Alex Ogle
So I’ve been on Humira for like, six years now. My rheumatologist brought up switching to a biosimilar last month, and honestly? I was terrified. Not because I didn’t trust the science - I’ve read enough to know the FDA doesn’t screw around - but because my body’s been stable for so long, and I didn’t want to risk anything. I mean, what if I started having flares? What if my skin broke out again? I spent a whole weekend scrolling through Reddit threads, watching YouTube videos from doctors, even calling my insurance. Turns out, the biosimilar cost me $120 a month instead of $580. I switched. No issues. Still feel like my old self. I didn’t even notice the difference. Weird, right? Like swapping out a battery in your phone and it still lasts just as long. Maybe that’s the real win here - not the savings, but the peace of mind that comes with knowing you’re not being nickel-and-dimed by a broken system.
Ryan Vargas
Let’s be real: the entire biosimilar narrative is a carefully orchestrated economic maneuver designed to shift financial burden onto the patient while maintaining the illusion of progress. The FDA’s approval standards? They’re not about safety - they’re about regulatory arbitrage. The fact that biosimilars can differ in minor structural ways but still be deemed ‘clinically equivalent’ is a mathematical trick, not a medical truth. We’re told they’re ‘highly similar,’ but similarity is a spectrum - and in biology, a single glycosylation difference can trigger an immune cascade. Why aren’t we seeing long-term immunogenicity studies beyond 52 weeks? Why are post-market surveillance systems so underfunded? The answer isn’t hidden in plain sight - it’s buried under layers of lobbying, patent thickets, and PBM rebates that ensure the original drug manufacturers still profit while patients are told to ‘just trust the data.’ This isn’t healthcare innovation. It’s financial engineering with a stethoscope.
Sam Dickison
Y’all are overcomplicating this. Biosimilars = cheaper version of the same drug. FDA says they’re good. Studies back it up. If your doc says switch, switch. No need to overthink it. I work in pharma logistics - I’ve seen the production lines. These things are made with insane precision. It’s not like they’re brewing it in someone’s garage. The real issue? Insurance policies. Some plans still push the brand because they get kickbacks. That’s not the biosimilar’s fault - that’s the system. Talk to your pharmacist. Ask if it’s interchangeable. If yes, you’re golden. If not, ask why. Either way, don’t let fear stop you from saving money. You’re not a guinea pig. You’re a patient who deserves affordable care.
Karianne Jackson
I switched to a biosimilar and my body just… gave up? Like, I felt like a ghost. Like I was walking around but not really there. I had zero energy. My joints ached like I hadn’t slept in a year. I cried for three days. I thought I was going crazy. Turns out, my body just needed the original. I went back. Now I pay more. But I feel like ME again. So yeah - maybe biosimilars work for some people. But not everyone. And nobody warned me. I felt like a lab rat. I hate this system.
Chelsea Cook
Oh honey, you’re telling me the system is broken? Newsflash - it’s been broken since 1998. But guess what? We’re fixing it. One biosimilar at a time. You think saving $1,750 a month on a drug that keeps you alive isn’t a win? That’s a whole vacation. A new laptop. A month’s rent. That’s dignity. That’s freedom. And yeah, some people have weird reactions - that’s biology. But 87% of users say they feel the same? That’s not a fluke. That’s progress. Stop being dramatic. The science is loud. The data is screaming. And if your insurance still won’t cover it? Call your rep. Write your senator. Tweet about it. We’re not waiting for permission to be healthy. We’re building the future. And it’s cheaper.
Andy Cortez
lol biosimilars are just a scam. They're gonna kill you. I read on a forum that one guy got cancer after switching. I don't trust the FDA. They're owned by big pharma. And why do they call it 'interchangeable'? Sounds like they want to swap you out like a battery. I'm sticking with Humira. Even if I have to sell my car. My life > their profits. Also, why is the government pushing this? Coincidence? I think not. #DeepState #PharmaControl
Jacob den Hollander
Hey, I just want to say - I’m a dad of two, and my wife’s on a biosimilar for RA. She was scared too. We sat down, talked to her doctor, looked at the studies together. We watched that NOR-SWITCH video. We called the Arthritis Foundation. She’s been on it for 14 months. No issues. Her pain is under control. Her insurance premium dropped. We’re saving $2,000 a year. That’s two extra weeks of childcare. That’s groceries without panic. I know it sounds small, but when you’re living paycheck to paycheck, that’s huge. I’m not saying everyone should switch. But if you’re scared? Talk to someone who’s been there. Find your tribe. You’re not alone. And yeah - your feelings matter. But so does the data. You don’t have to choose between fear and facts. You can hold both. And still move forward.
Kathryn Lenn
Wow. So we’re supposed to just trust the FDA? And the ‘studies’? Who funded them? Who wrote them? Who owns the patents on the biosimilars? Hint: it’s usually the same company that made the original drug. This isn’t innovation - it’s rebranding. They’re just repackaging the same monopoly with a new label. And now they want you to believe you’re saving money? Please. The rebates are still going to the same middlemen. The real savings? They’re going into CEO bonuses. I’ve seen the spreadsheets. This isn’t healthcare reform. It’s corporate theater. And you’re the audience.
John Watts
Look - I’ve worked in clinics for 18 years. I’ve seen patients cry because they couldn’t afford their meds. I’ve seen people skip doses. I’ve seen people die because they chose between rent and rheumatology. Biosimilars? They’re not perfect. But they’re the best thing we’ve got right now. I’ve prescribed them. I’ve watched people get their lives back. One guy? He started painting again after 10 years. Another? Went back to work. That’s not just a drug. That’s hope. Yeah, the system’s messy. Yeah, insurance is a nightmare. But if you can switch - and you’re stable - do it. Talk to your pharmacist. Ask questions. But don’t let fear stop you from living. You deserve to feel better. And sometimes, better doesn’t mean the same. It means cheaper. And that’s okay.